By the early nineteenth century, chemists were striving to organize their rudimentary knowledge of the chemical elements. It was known that differing masses of elements reacted to form compounds. For example, they found that 3 grams of magnesium metal reacted with precisely 2 grams of oxygen to form magnesium oxide with no residual magnesium or oxygen. The same mass of oxygen, however, required 5 grams of calcium metal to react completely to form calcium oxide. Table 1 summarizes these relative combining masses.
Chemists gradually discovered that such relative masses in chemical reactions were fundamental characteristics of the elements. The English chemist John Dalton realized that all the known combining masses were nearly whole‐number multiples of the combining mass of the lightest element—hydrogen. In 1803, he proposed an atomic theory in which all other elements would be built from multiple hydrogen atoms. Consequently, he based his scale of atomic masses on hydrogen being equal to 1.
Although Dalton's theory was found to be unrealistically simple, he did compel chemists to adopt a standard scale of atomic weights. Because the combining mass of oxygen is approximately 16 times that of hydrogen, the preceding chart can be revised, as shown in Table 2.
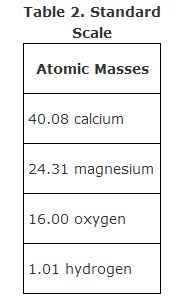
The modern masses for calcium, magnesium, and oxygen are still nearly in the 5:3:2 ratios of the original masses. Notice especially that the atomic mass of hydrogen is not precisely equal to 1, because the atomic mass scale is now based on the most common variety of carbon being exactly 12 atomic mass units. Dalton's bold conjecture that all the heavier elements have masses that are integral multiples of hydrogen is not strictly valid, but his theory was a good approximation that eventually led to the discovery of the particles composing the atoms.
|
|
|
|
|