Eukaryotic genetic replication involves both DNA synthesis and chromatin assembly. Chromosomal DNA synthesis is similar to prokaryotic DNA replication in that each of the two strands serves as template for new synthesis. In contrast to the situation in prokaryotes, eukaryotic DNA replication is limited to a single portion, the S phase, of the cell cycle. The cell cycle of eukaryotic cells is divided into distinct phases. M phase, which is the phase where mitosis takes place, is the “start” of the cycle. After cell division, a “gap” phase, G1, commences, in which enzyme synthesis and metabolism take place. G1 can last for a very long period of time, and many cells in animals are “arrested” in G1 for years without dividing. Controlling G1 arrest is clearly important for understanding cancer, which is essentially a disease of replication control. The movement from G1 to S phase (the DNA replication phase) commits the cell to dividing. After S phase, a second gap exists, G2, which lasts until the beginning of mitosis. The biochemical reactions that govern these events involve proteins that are made and then broken down at specific points of the cell cycle. These proteins, called cyclins, are kinases that phosphorylate other proteins in the cell, leading eventually to the start of chromo‐somal DNA replication. At other phases of the cell cycle, other cyclin‐type kinases control the entry into mitosis and the various phases of mitosis itself. See Figure 1 .
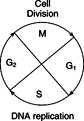
Figure 1
Unlike prokaryotes, where DNA replication begins at a single origin, eukaryotic cells use multiple origins of replication to initiate bidirectional synthesis. The replication forks appear to be attached to the nuclear membrane at distinct sites; the replicating chromatin may be pulled through these sites. A number of DNA polymerases exist in the nucleus. Activation of replication involves the assembly of one form of the enzyme, polymerase δ, with a special subunit called proliferating cell nuclear antigen (PCNA) so that it is capable of synthesizing long chains of DNA without falling off the template strand. As in prokaryotic DNA replication, continuous and discontinuous replication occurs on the leading and lagging strands respectively, so that the overall process is semiconservative. See Figure 2 .
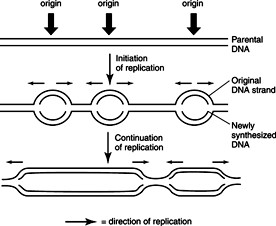
Figure 2
Nucleosome assembly differs from DNA replication. Histone content of a cell doubles during cell doubling, just as the DNA content does. On the leading strand, the pre‐existing histone octamers briefly dissociate from the template and then re‐bind to the double helix. Newly made nucleosome core particles associate with DNA on either strand. Thus, the overall process of histone doubling is conservative (the histones stay together during replication), in contrast to DNA synthesis, which is semiconservative.
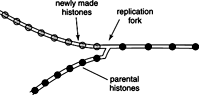
Figure 3
The end of a linear chromosome is called a telomere. Telomeres require a special mechanism, because the ends of a linear chromosome can't be replicated by the standard DNA polymerases. Replication requires both a template and a primer at whose 3′ end synthesis begins. The primer can't be copied by the polymerase it primes. What copies the DNA complementary to the primer? In a circular chromosome, the primer site is to the 3′ direction of another polymerase, but in a linear chromosome, no place exists for that polymerase to bind. As a result, unless a special mechanism for copying the ends of chromosomes is used, there will be a progressive loss of information from the end of the linear chromosome. Two characteristics about telomeres help avoid this situation. First, they consist of a short sequence—for example, AGGGTT—repeated many times at the end of each chromosome. Telomeres, therefore, are part of the highly repetitive DNA complement of a eukaryotic cell. Secondly, a specific enzyme, telomerase, carries out the synthesis of this reiterated DNA. Telomerase contains a small RNA subunit that provides the template for the sequence of the telomeric DNA. Eukaryotic somatic cells have a lifespan of only about 50 doublings, unless they are cancerous. One theory holds that a lack of telomerase in cells outside the germ line causes this limitation.