The original nucleus is called the parent nucleus, and the nucleus remaining after the decay is called the daughter nucleus. The process of one element changing into another through radioactivity is called transmutation.
If a nucleus emits an alpha particle, it loses two protons and two neutrons; therefore, the daughter nucleus has an atomic mass of 4 less and an atomic number of 2 less than the parent nucleus. An example of alpha decay of uranium is 92 238U → 90 234Th + 2 2He.
If a nucleus emits a beta particle, it loses an electron. Since the mass of the electron is so small compared to that of a proton and a neutron, the atomic mass of the parent nucleus is the same as the daughter nucleus. The atomic number of the daughter nucleus is one greater than that of the parent nucleus. An example of beta decay of bismuth is 83 212Bi → 84 212P 0 + − 0 e.
Frequently the daughter nucleus is left in an excited state after either alpha or beta decay. Then the nucleus can give up excess energy by emission of gamma radiation. The following example shows a typical situation where gamma decay occurs: 5 12B → 6 12C* + −1 0 e; then, 6 12C* → 6 12C + γ, where the asterisks indicate an excited nucleus.
The rules for radioactive decay are based on conservation laws. Examination of the preceding examples reveals that the number of nucleons and the electric charge are conserved; that is, the total on one side of the equation equals the total on the other side of the equation. Other conservation laws that must be observed are those of energy, momentum, and angular momentum.
Half-life
The decay rate ( R) or the activity of a sample of radioactive material is defined as the number of decays per second, given by R = − λ N, where N is the number of radioactive nuclei at some instant and λ is the decay constant.
The half‐life ( T) is defined as the time required for half of a given number of radioactive nuclei to decay. It is different for each type of radioactive element:
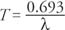
The general decay curve for a radioactive sample relating the number of nuclei present at a given time to the original number of nuclei is exponential. The expression is 0 1 n + 92 235U → 56 241Ba + 36 92Kr + 3 0 1 n. The total rest mass of the products is less than the original rest mass of the original uranium by 220 MeV. This is an enormous amount of energy compared to energy releases in chemical processes and when considering that a relatively modest piece of uranium has so very many nuclei. Nuclear fusion occurs when light nuclei are combined to form a heavier nucleus. The sun is powered by nuclear fusion.
The binding energy is related to stability. When the mass energy of the parent nucleus is greater than the total mass energy of the decay products, spontaneous decay will take place. If the decay products have a greater total mass energy than the parent nucleus, additional energy is necessary for the reaction to occur. Energy is released when light nuclei combine (fusion) and when heavy nuclei split (fission).